Rate of Reaction: Formula, Examples, Calculation units & Factors
How can you determine the Rate of Reaction?
The Rate of Reaction can be determined by the following two methods:
Physical Methods
In these methods, we measure the change of some physical property of reactants or products. The following are the methods generally used:
| Physical Methods | Observed Physical Property | |
| 1. | Refractometric method | Change in refractive index. |
| 2. | Spectroscopic Method | Absorption of ultraviolet or infrared radiations. |
| 3. | Calorimetric method | Change in color intensity. |
| 4. | Conductivity method | Change in electrical conductivity. |
| 5. | pH method | Change in pH observed. |
| 6. | Polarimetric method | Change in the optical rotation of plane-polarized light. |
Chemical Method
In this method, generally, samples are drawn from the reacting vessel at regular intervals of time. The reaction is stopped by chilling the sample by adding a suitable chemical. The number of reactants or products can be determined by titrating the samples with a proper chemical reagent.
CH3COOH + H2O ↔ CH3OH + CH3COOH
The titration is followed by measuring of acetic acid by titrating the sample of the regular intervals of time with standards alkaline (NaOH).
With the passage of time, more and more acetic acid is produced. Then, a graph is plotted between concentration and time and the rate of reaction is calculated by the graph.
6 Factors Affecting the Rate of Reaction
The rate of reaction is affected by the following factors: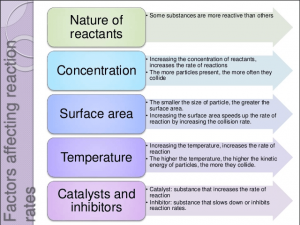
- The concentration of reactants.
- Nature of reactants.
- Temperature
- Presence of catalyst.
- The surface area of reactants.
- Radiation
Concentration of Reactants
the rate of reaction varies with the concentration of the reactants.
Rule
According to the law of mass action rate of reaction is directly proportional to the concentration of reactants.
Effect
The greater the concentration of the reactants, the higher the rate of reaction.
Reason
If the concentration of the re4actant increases, so, the number of colliding reactant molecules also increases, which increases the frequency of collision between them. Hence, the rate of reaction increases.
Effect of Temperature
The rate of reaction varies with temperature.
Effect of Exothermic Reaction
In an exothermic reaction, the rate of reaction decreases with the increase in the temperature.
Effect on Endothermic Reaction
In endothermic reactions, the rate of reaction increases with the increase in temperature.
Rule
It has been observed that for every 10K rise in temperature, the rate of the reaction becomes double.
Reason
- If the temperature increases, the molecular velocity increase, so, the frequency of the collision increases, hence, the rate increases.
- It has been observed that for every 10K rise in temperature, the number of effective collisions becomes double, hence, the rare is also doubled.
Milk sour more rapidly in summer than winter
The rate of reaction is affected by temperature. By increasing temperature the kinetic energy of the molecule increases. Hence, the rate of reaction increases. The sour taste of milk is produced during a chemical reaction. In summer the temperature of the environment is greater as compared to winter that is why in summer spoiling of the milk proceeds much more rapidly than in winter.
Food kept in refrigerators can be stored for a longer
It is because of the rate of decomposition of food various directly with temperature which is quite low in refrigerators. Hence, the decomposition of food becomes slow waste and so the food kept in the refrigerator can be stored for a longer time.
Effects of Surface Area of Reactants
The rate of reaction varies with the surface area of reactants.
Effect
The greater the surface area of reactants, the higher is the rate of reaction.
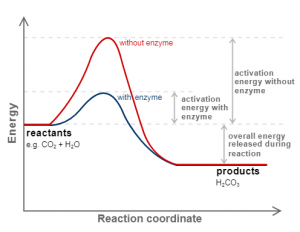
Effect of Catalyst
It is the substance that alters the rate of the reaction without being used in the chemical reaction.
Effect of Nature of Reactants
The rate of reaction varies with the nature of reactants.
Reason
The activation of energy for the effective collision of the different substances is different.
Effect
The rate of reaction having high activation energy is low.
Effect of Radiation
The sunlight contains ‘photons’ when these high energy photons strike the reactants molecules they provide the necessary activation energy to them for a reaction.
Activation Energy
It is the extra amount of energy that the reactant molecules have to absorb so it’s internal energy becomes equivalent to threshold energy.
Mathematical formula
Activation energy = Threshold energy – Average Internal Energy