20 Types of Amino Acids with Definition and Classification
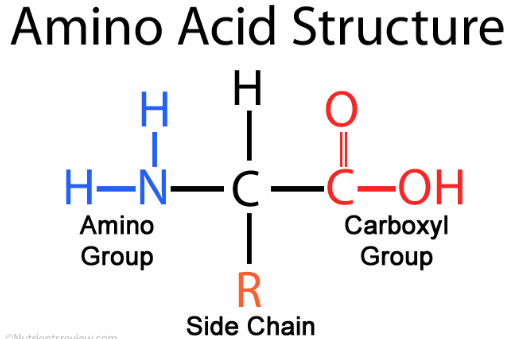
Amino acids are organic compounds that contain an amino group (-NH2), carboxyl group (-COOH) and a side chain called functional group that is specific to each amino acid.in common, 20 types of amino acids are preferred which are further grouped into 3 major types such as essential amino acids, non-essential amino acids, and conditional amino acids.

Let’s Read more About in detail:
What are amino acids?
Amino acids are carboxylic acids having amino groups. the most important types of amino acids are the α-amino acids, Amino acids are mostly formed with carbon, nitrogen, oxygen, and hydrogen along with other naturally occurring elements. about 500 amino acids are known to us in which 20 are the most important and common in use.
they are present as the second-largest component in the human body in making muscles and tissues along with water.
the general formula for Amino Acids is given bellow in the diagram.
Amino acid Structure & Formula
hundreds of amino acids are possible, but only twenty are found in proteins and all of these α-amino acids.
20 Types of Amino Acids List
20 common Amino acids are given below in the form of the list along with three-letter and amino acids symbols one-letter code (upper-case) in which there are often known to us. these one letters are used for the quick notation of amino acids and are easy to learn.
- alanine – ala – A
- arginine – arg – R
- asparagine – asn – N
- aspartic acid – asp – D
- cysteine – cys – C
- glutamine – gln
- glutamine – gln – Q
- glutamic acid – glu – E
- glycine – gly – G
- histidine – his – H
- isoleucine – ile – I
- leucine – leu – L
- lysine – lys – K
- methionine – met – M
- phenylalanine – phe – F
- proline – pro – P
- serine – ser – S
- threonine – thr – T
- tryptophan – trp – W
- tyrosine – tyr – Y
- valine – Val – V
Essential Amino acids
These are the amino acids, which cannot be synthesized in our body but they are essential for:
- The growth of infants.
- Transmission of impulses in the nervous system.
Their deficiency results in many diseases. They must be added to our bodies through our diet. Examples are:
- Leucine
- Iso-Leucine
- Methionine
- Threonine
- Arginine
- Valine
About 10 Amino acids are essential.
Classification of Amino Acids
Amino acids have been classified according to their natural, acidic or basic nature.
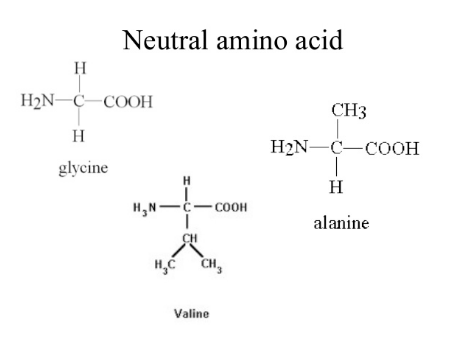
Neutral Amino acids
These exhibit amphoteric nature. They contain one basic (-NH2) and one acidic (-COOH) group.
Acidic Amino Acid
They exhibit acidic nature. They contain one basic (-NH2) group and more than one acid (-COOH) group.
Basic Amino acids
They exhibit basic character. They contain one acidic (-COOH) group and more than one basic Amino (-NH2) group.
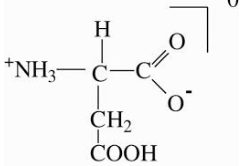
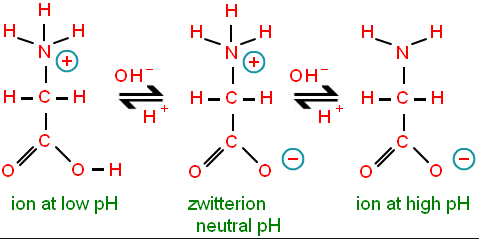
Zwitterion (Dipolar nature)
A dipolar charged but overall electrically neutral ion is called Zwitterion. In an amino acid, the carboxylic (-COOH) group ionizes to donate a proton (H+), whereas the amino (-NH2) group with a lone pair of electrons behaves as a proton accepter i.e. Lewis base. Hence amino acid exists more as dipolar ion i.e. Zwitterion than in an unionized form.
The most important reaction of the amino acid is the formation of peptide bonds and this is possible because amino groups of one acid can react with a carboxylic group of another and vice versa.
Although there is only one peptide bond in the above compound, it is called a dipeptide.
Types of amino acids(core structural functional groups)
- Those having an amino (-NH2) group on alpha (α) are called α-Amino acids.
- Those having an amino (-NH2) group on beta (β) are called β-Amino acids.
- Those having an amino (-NH2) group on gamma (ϒ) are called ϒ -Amino acids.
Rol in the Human body
The role of Amino acids in the human body is as follows:
- Amino acids may be synthesized back into protein.
- Oxidation may take place to provide energy.
- If the diet is low in carbohydrates or fats, body protein may be transformed into either of these or used to make hormones and other body necessities.