Importance of Carbon in Biochemistry
Carbon is the basic element of organic compounds. Due to its unique properties, carbon occupies the central position in the skeleton of life. So, it has a great importance for living things. Carbon shows following properties:
[wp_ad_camp_1]
1. Carbon a tetravalent
Carbon is tetravalent. It can react with many other known elements forming covalent bonds.
2. Carbon a tetrahedron
When a carbon atom combines with four atoms or radicals, the four bonds are arranged symmetrically in a tetrahedron, and result to give a stable configuration. The stability associated with the tetravelancy of carbon atoms make sit a favourable element for the synthesis of complicated cellular structures.
3. Catenation
Carbon atoms can also combine mutually forming stable, branched or unbranched chains or rings. This ability of carbon is responsible for the vast variety of organic compounds. C-C bonds form a skeleton of organic molecules as shown below;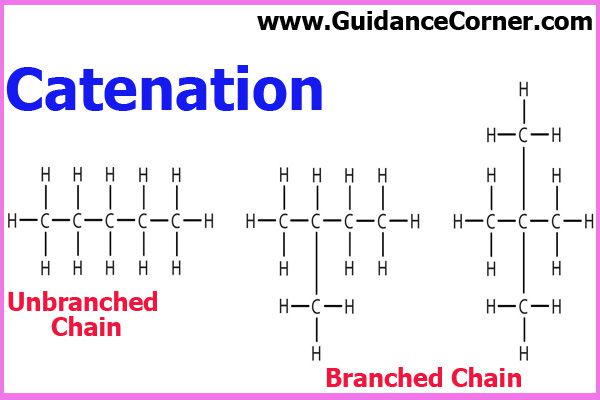
4. Combination of Carbon with other Elements
Carbon combines commonly with the other elements like H, O, N and S. it forms many types of compounds with these elements:
v Carbon Hydrogen Bond (C-H):
This C-H bond is a source of energy for the cellular activities.
[wp_ad_camp_2]
v Glycosidic Linkage:
Carbon oxygen association (C-O) forms glycosidic linkage in carbohydrates. So, carbohydrates are stable compounds.
v Peptide Linkage:
Carbon combines with the nitrogen in amino acid linkage. The bond formed between two amino acids is called peptide bonds. Protein is formed by peptide bond. Proteins have different structures and functions. Therefore, proteins play an important role in the cell.
v Synthesis of Macromolecules:
Cellulose, fats, proteins, etc. are large organic molecules. They are insoluble in water. They form many structures of the cells. They also act as storage compounds. They break structures of the cells. They also act as storage compounds. They break and release small molecules like glucose. Glucose provides energy to the body.
v Synthesis of Micromolecules:
Glucose, Amino Acids, Fatty Acids, etc. are small organic molecules. They are subunits of the macromolecules. Some small molecules are unstable. They break and release energy, e.g. ATP. Such substances are immediate source of energy for the cellular metabolism.
Importance of Water in Biochemistry
Water is tasteless, odorless and nearly colorless with blue hint. Water is a medium of life. It is the most abundant compound in all organisms. It varies from 65 to 89 percent in different organisms. Human tissues contain about 20% water in bone cell occur in the presence of water. It also takes part in many biochemical reactions such as hydrolysis of macromolecules. It is also used as raw material in photosynthesis.
[wp_ad_camp_3]
1) Role of Water in Chemical Reaction
All the reactions take place in the presence of water. It takes part in many biochemical reactions. For example, water causes hydrolysis of macromolecules. Water is also used in photosynthesis.
2) Solvent Properties
Water is a polar or ionic molecule. So it is an excellent solvent for polar substances.
- Ionic substances dissolve in water and dissociate into positive and negative ions. Ionic compounds have charged groups in their molecules. So, they disperse in water. Ions and molecules move randomly in solution. Therefore, they can react easily with other molecules and ions. Due to this property, all reactions occur in the water medium in the cell in the presence of enzymes. These enzymes act only in aqueous environment.
- Non-polar molecules like fats are insoluble in water. So, these compounds form membranes of cells.
3) Heat Capacity
The number of calories required to raise the temperature of 1g of water from 15 to 16 is called specific heat capacity of water. The specific heat capacity of water is 1.0. Water has a great ability to absorb heat without much change of its own temperature. So, water acts as temperature stabilizer for the organism in the environment. It protects the living material against sudden thermal changes.
OR
The specific heat capacity may be defined as the amount of energy needed to raise the temperature of a fixed amount of a substance by 1. It takes 100 to boil and similarly, loses temperature very gently. This stability of temperature of water plays an important role in cell.
[wp_ad_camp_4]
4) Heat of Vaporization
The amount of heat required to change 1gm of water into vapours is called heat of vaporization, or specific heat of vaporization. It is expressed in calories per grams. The specific heat of vaporization of water is 547 Kcal\kg. So, water absorbs much heat when changes from liquid to gas. Thus water regulates heat produced during oxidation. It also cools the plants during transpiration. It cools the animals during evaporation of water. The evaporation of only 2ml of water from a liter of water lowers the temperature of remaining 998ml water by 1.
5) Ionization of Water
The water molecule ionizes to form and ions. This reaction is reversible. But, equilibrium is maintained. The concentration of and ions is about mole/liter in pure water at 25. The ions affect and take part in many reactions in cell.
This reaction is reversible. But, equilibrium is maintained. The concentration of and ions is about mole/liter in pure water at 25. The ions affect and take part in many reactions in cell.
6) Protection
Water is a lubricant. It reduces friction. Thus it protects the body parts from damage. For example, tears protect the eye from the rubbing by eyelids. Water form a protective layer of fluid around the organs and protects them from trauma.
7) Water act as Amphoteric Molecule
Water acts both as acid and base. As acid it gives up an electron to form H+ and as base it gains electron to form OH- ions.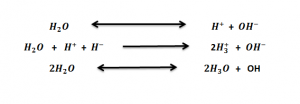 This ability of water helps in quick change of medium for the activity of enzymes.
This ability of water helps in quick change of medium for the activity of enzymes.
8) Cohesive Force
It has cohesive force due to presence oh H ions. This force of cohesion helps the plants in Ascent of sap and conduction of various materials through the vascular bundles.
9) Chemical Formula
It is DIPOLIC in nature. It has very slightly +ve end, the hydrogen and slightly –ve end, the oxygen atom. The most significant result of this charge separation is the tendency of water molecule to form hydrogen bonds.