Hydration Energy Definition & Meanings : A Complete Guide
Hydration of ions is an exothermic process. The energy released when one mole of an ion in the gaseous state is dissolved in water to get it hydrated is called hydration energy. It is the energy released in the hydration of M+(g) ions.
M+(g) + aq → [M(aq)]+ + Energy released
This energy highly depends on:
(i) ionic radius and
(ii) Charge on the ion.
In other words, this energy mainly depends on the charge to size ratio of the ion. Since, the charge to size, the ratio decreases from top to bottom in a group, It also decreases from top to bottom in a group.
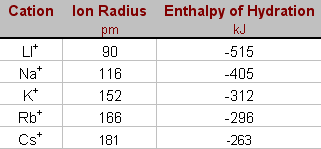
Thus, this energy decreases from Li+ to Cs+ in group IA. The energy increases significantly by moving from left to right in a period as the charge to size ratio increases, as observed in the metal ions of the 3rd period.
For example, the hydration energy is greater for Mg2+ than for Na+, because Mg ion has a large charge, as well as being a smaller ion than Na+.
The negative sign associated with the hydration energy values indicates the release of energy.
Hydration Energy of ions ( ΔHhyd) in kJ mol-1
| IA | IIA | IIIA | VIIA | |
| Period | Li+ | Be2+ | B3+ | F– |
| -515 | -1757 | -457 | ||
| Na+ -406 K+ | Mg2+ -1450 Ca 2+ | Al3+ -4613 | Cl– -384 Br– | |
| -322 | -1145 | -351 |
Trends in Alkali metals
The extent of hydration energy trends in Alkali metals depends upon the size of the ions that participated in metals. if the size of the ions is greater, It will be smaller and vice versa. you can understand better by this sequence.
Trends in Alkaline earth metals
The hydration enthalpy becomes smaller as the size of the ion increases in alkaline metals and vice versa as shown in the figure.


Interesting read! I had no idea that hydration energy was a trend in the periodic table. It’s fascinating to see how the electrons in an atom are responsible for transferring energy and how this affects the overall hydration energy of an element. Looking forward to more insights on this topic!