Characteristics of Colloidal Solutions with Examples
What are Colloidal Solutions?
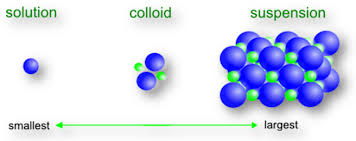
A colloidal solution is a heterogeneous mixture in which solute particles are larger than molecules or ions, but not large enough to be seen by the naked eye. For example, clay in water or starch in water. A colloidal solution is also called a false solution.
In a colloidal solution, the solvent is more appropriately called the dispersion medium (water) while the solid solute particles constitute dispersed medium (clay or starch).
Characteristics of Colloidal Solutions
The colloidal solutions exhibit the following properties:
- The colloidal particles are not large enough to be seen with the naked eye.
- The colloidal particles are formed by a large collection of molecules clinging to one another.
- The solution does not exert any observable osmotic effect.
- The solution cannot be dialyzed, i.e., the solutes cannot diffuse through a semi-permeable membrane.
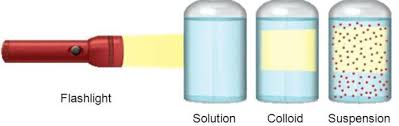
- The solutes can scatter light rays. i.e., the solution exhibits the Tyndall effect.
Colloidal Systems
The term colloid was first suggested by Graham in 1861 and is derived from two Greek words kolia=Glue + eiods=Like. The term was used to describe glue-like preparations such as solutions of proteins and liquid preparations of vegetation gums, e. g. gum arabic. However, there are many colloidal suspensions that are not glue-like.
Types of Colloidal Systems
The colloidal systems can be classified into two types generally:
Sol: The colloidal systems with the property of fluidity
Gels: The colloidal systems without the property of fluidity. The gels are actually
Sols that adopt a semi-solid or semi-rigid form, e. g… fruit jellies.
Classification of Colloidal Systems
Colloidal systems in liquids are more common and important. On the basis of their Affinity for liquids the colloidal systems can be classified into two general classes:
Lyophilic Systems:
Such systems in which the dispersed phase and liquid dispersion medium are attracted to each other.
Lyophobic Systems:
The systems in which the dispersed phase and liquid dispersion phase repel each other.
Hydrophilic and Hydrophobic Systems
If the dispersion medium is water then the terms hydrophilic (water-loving) or Hydrophobic (water-fearing) are used respectively instead of Lyophilic or lyophobic.
- Hydrophilic Systems: If starch, gelatin, or agar is added to hot water rare, quantities of water are taken up by these colloidal substances hydrophilic systems. The particles of the dispersion medium are adsorbed on the surfaces of colloids in such systems.
The hydrophilic colloidal sols are more important in cell physiology. Many cell contents and cell products such as protoplasm, proteins, starch, cellulose, resins, and gelatin are strongly hydrophilic and form hydrophilic colloidal sols.
- Hydrophobic Systems: The hydrophobic colloids are generally compounds of inorganic nature and in most cases, they are more difficult to prepare than are hydrophilic colloids. The common examples of hydrophobic systems are gold sol and rubber dispersed in water.
Properties of Biological Importance & Systems
The colloidal systems exhibit the following properties:
- Tyndall Effect: If a narrow beam of light is passed through a colloidal system its path can be observed because the colloidal particles scatter light. This is called the Tyndall effect and the path of light that appears as a cone is known as Tyndall. This property of colloidal particles enables us to detect the presence of colloidal particles.
- Brownian Movements: Robert Brown, a Scottish Botanist in 1927 observed that colloidal particles exhibit random dancing hops per second. These movements were named Brownian movements. These movements are due to the bombardment of different sides’ colloidal particles by water molecules and prevent colloids from settling down.
- Adsorption: Colloidal particles exhibit a high tendency of adsorption (the tendency of molecules and ions to adhere to the surface of certain solids or liquids) which helps increase surface area. Thus, colloidal systems provide a large surface area for adsorption of molecules and ions. ( See Also: Difference between Absorption and Adsorption)
- Electrical Properties: Colloidal particles of a colloidal suspension carry the same electric charge, either positive or negative (clay particles). These charges result from the adsorption of free ions in the dispersion medium. As a result, an electrical double layer is produced around the colloidal particles.
- Precipitation: if an electrical double layer present around the colloidal particles is removed by the addition of an electrolyte, the dispersed particles of a colloidal suspension will aggregate and finally precipitate.
- Phase Reversal: Certain lyophilic sols form an extremely viscous solid-like mass called gels under certain conditions. For example, aqueous gelatin or agar sols will set when cooled and will form a jelly-like mass. The conversion of a soul to a gel is called gelation. If a gel of gelation. If a gel of gelatin or ager is heated, it will convert back to a sol, a process known as solation. The property of colloidal dispersion is called phase reversal.
- Filtration: The colloidal particles cannot pass through a parchment membrane or collodion like the particles of a true solution. However, the colloidal particles cannot be separated from the dispersion medium with ordinary filter paper. This property of colloidal dispersions is used to separate them from true solutions by a process called dialysis.
Biological Importance of Colloidal Solution & Systems
Protoplasm a Colloidal Solution
The protoplasm exhibits almost all the properties of colloidal solutions such as:
- The soluble fraction of the cytoplasm exhibit this Tyndall effect indicating, the presence of colloidal particles in it.
- The dispersed phase of protoplasm shows Brownian movements depicting the colloidal nature of the protoplasm.
- The cyclosis is duo to phase reversal colloidal property of the protoplasm. The cyclosis usually occurs in the sol phase and s affected by hydrostatic pressure, temperature, pH, viscosity, etc.
- Protoplasm exhibits buffering property of colloidal systems. Different pH has been recorded in different regions of the CET. For example, the pH of vacuolar sap is either basic or acidic whereas the pH in aqueous nucleoplasmic matrix ranges from 7.6 to 7.8. The PH of the protoplasm is liable to constant changes due to the production of acids and bases during the metabolic processes occurring in the cell protoplasm. The cytoplasm contains buffering systems such as carbonate-bicarbonate systems which helps in maintaining the pH of the cell protoplasm.
- The protein molecules with their large surfaces provide adsorptive surfaces in the protoplasm. This colloidal property also forces the cytoplasmic matrix to form the protein boundaries.
- The polarity of the egg is determined by the cell-matrix because when certain eggs have been centrifuged the cell components become stratified hut original polarity s maintained. This property is also due to the stable colloidal nature of the cytoplasmic matrix.