Structure of Ionic Compounds with Definition and Examples
Structure of Ionic Compounds: There are four structures that account for a large number of the 1:1 ionic solids. These are NaCl, CsCl, zinc blende, and wurtzite. The mineral zinc blende and wurtiz1te both consist of ZnS, but they have different structures.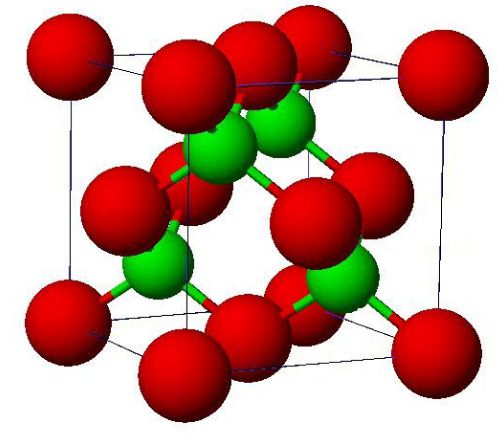
Structure of Ionic Compounds
Sodium Chloride Structure
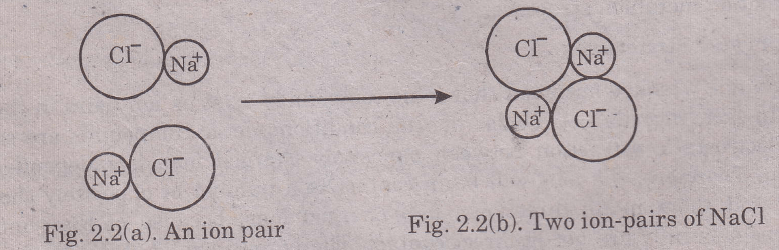
An “ion pair” of sodium chloride can be represented as shown in Fig. 2.2, When a second ion-pair is brought near to the first, the two · will attract each other and arrange themselves as shown in Fig. 2.2 (b). In the [Na+Cl-] configuration there is an additional attractive force of each Na+ ion for the CC ion of the other pair.
The attractive forces between the oppositely charged ions are much greater than the repulsive forces between the like ions (Na+ and Na+ or Cl- and CC). As a result of this, the energy of the system would decrease.
If more than two ‘ ion pairs ‘ come together, ‘a three-dimensional lattice is built up. If four ‘ion-pairs’ come together, a cubic arrangement is formed (Fig. 2.3). It constitutes the unit cell for NaCl. The three ‘ dimensional model of NaCl crystal based upon the unit cell (Fig. 2.3) is shown in Fig. 2.4.
The ratio of the ionic radii in the NaCl is 0.53, which suggests an octahedral arrangement in a cubic closest packed structure. Since an octahedral site is formed by six spheres, each Na+ ion (small sphere) is surrounded by six Cl- ions (large spheres). In turn, each CC ion is
surrounded by six Na+ ions. We can see that the coordination number of Na+ is 6 and that this ion sits in an octahedral hole defined by the six nearest Cl- ions.